TCR service offerings
- Dedicated team of scientists with over 20 years of experience and expertise in performing TCR screening
- Experience and expertise in developing protocols for novel antibody classes including: murine, humanized, chimeric, fully human, antibody cocktails, Fab/VH fragments, fusion proteins, bispecific superantigens, biomers, tetramers and nanobodies, as well as variable regions attached to various carrier backbones
- Custom antibody/protein labeling services available
- Dedicated and experienced Scientists to develop and optimize IHC staining protocols to generate robust and meaningful cross reactivity data
- Experienced study directors with extensive histology and IHC knowledge to advise and guideLicensed facilities with access to high quality frozen human and animal tissues covering both FDA and EMA regulatory guidelines
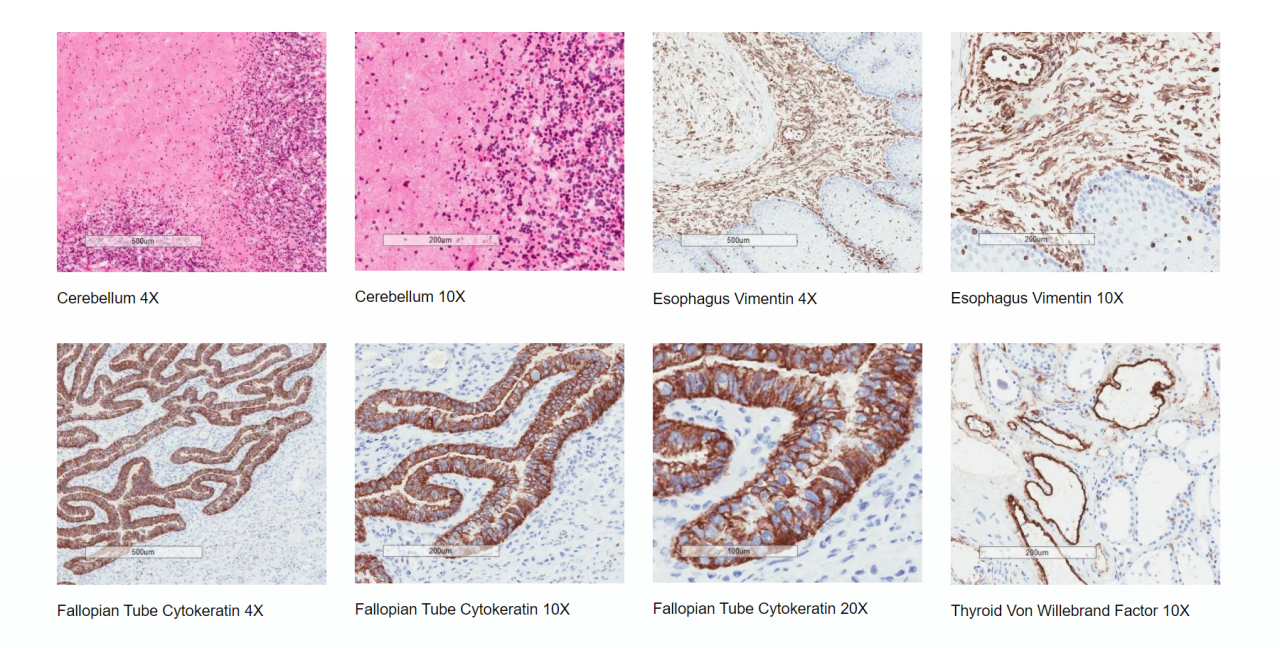
- Assessment of tissue suitability for each study to ensure tissue and antigenic integrity (see images below)
- Manual and automated IHC staining platforms available, allowing flexibility and customization of IHC methods
- Expert Pathologists (MRCPath, FRCPath, American Board Certified) with experience in evaluating and interpreting staining from TCR assessments
- Flexible reporting criteria to ensure study endpoints are met